Set 5, 2024
Gene therapy is a medical approach that aims to prevent and treat diseases by the use of genetic material. It adds a completely new gene, or a modified one to the person. It can even replace the genes with other genes, or it can inactivate genes. That way, gene therapy aims to get rid of a disease by replacing or inactivating a bad gene.
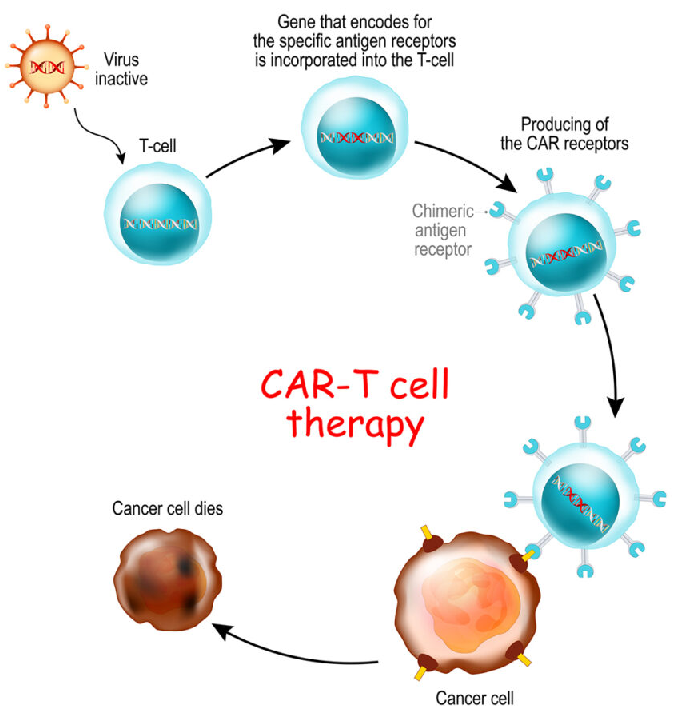
Gene therapy can potentially treat genetic disorders, also can be used for Cancer therapy. This is because it introduces genes that kill cancer cells, or help the immune system fight cancer. Also: it can be used for the treatment of infectious diseases, as it can introduce genes that make the cells resistant to viruses or bacteria.
Steps to Gene Therapy Process:
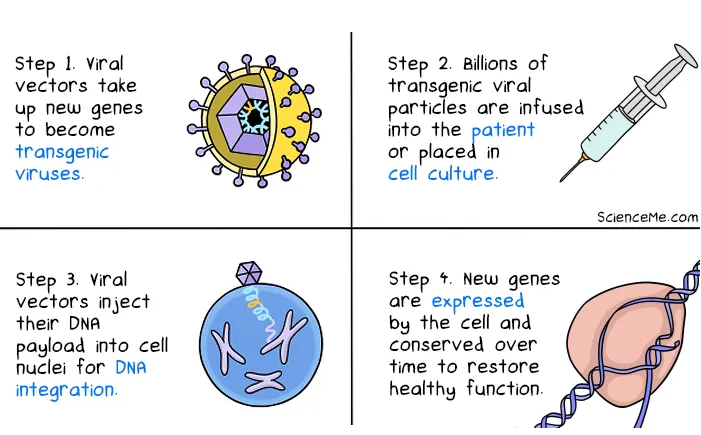
The first step of gene therapy’s process is gene identification. This pinpoints the gene, or the lack of the gene that is responsible for the disease.
The second step is the creation of a therapeutic gene,creating the gene that will replace the unhealthy gene or the lack of gene. The third step is the gene delivery, with some way the gene is introduced inside the body using biological vehicles, such as viruses. When this process ends the gene enters and integrates into the DNA of the cells and the gene starts to produce the expected protein.
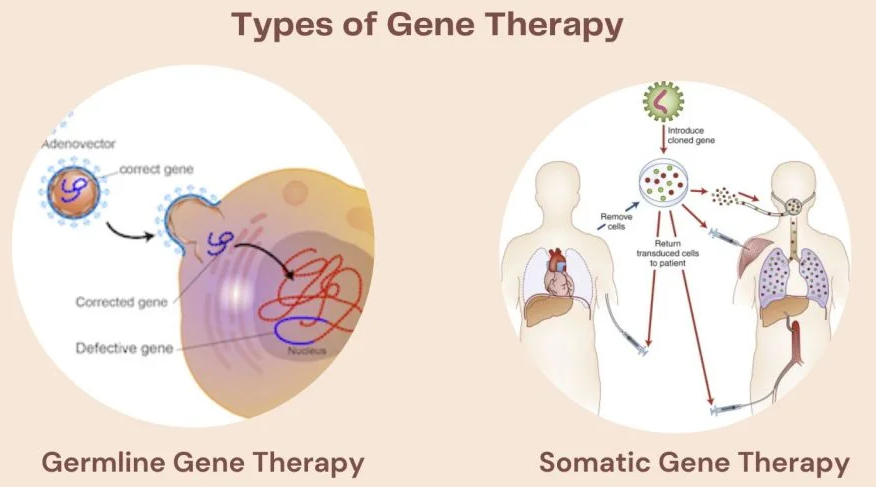
There are two types of gene therapy - somatic gene therapy, which modifies genes in non-reproductive cells, and germline gene therapy, which modifies reproductive cells. This means that somatic gene therapy affects who has gene changed and germline gene therapy affects the next generations descended from who was modified.
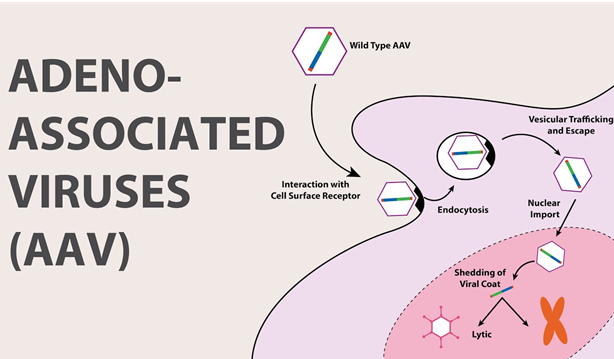
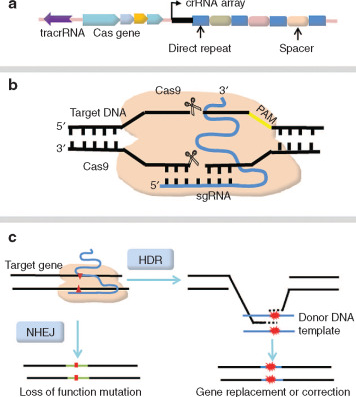
The gene delivery in the case of gene addition, very often uses an adeno-associated virus (AAV) for the delivery. In the case of gene silencing, it targets the messenger RNA, decreasing the amount of a certain protein. On the other hand, gene editing uses a genetic material which was delivered to edit or change pieces of DNA, using some technology like CRISPR-Cas9.
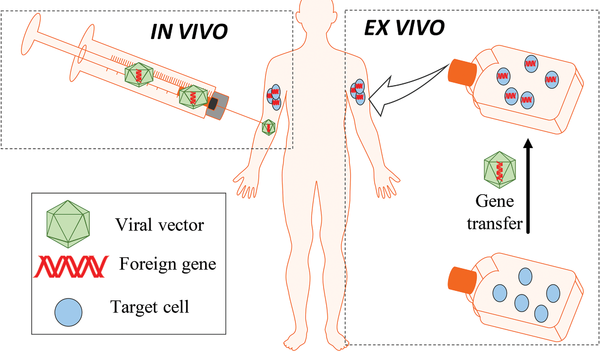
The gene delivery can be done in two different ways in your body. It can be done In vivo. This means the gene is inserted into your body, using for example an injection or viruses; or Ex vivo, which means that after the removal of your cells and the delivery of the gene of those cells outside of your body the modified cells return to your body.
There are some vectors that bring the DNA package to the body, they can be viral vectors and non-viral vectors, but the non-viral vectors are still under research and it can be a lower cost and lower immune response alternative intending to deliver large DNA packages.
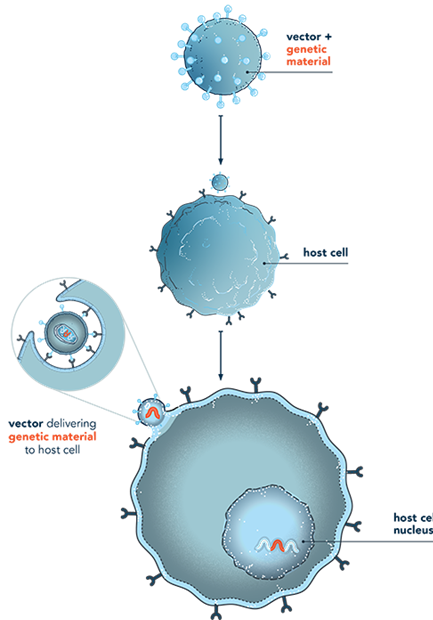
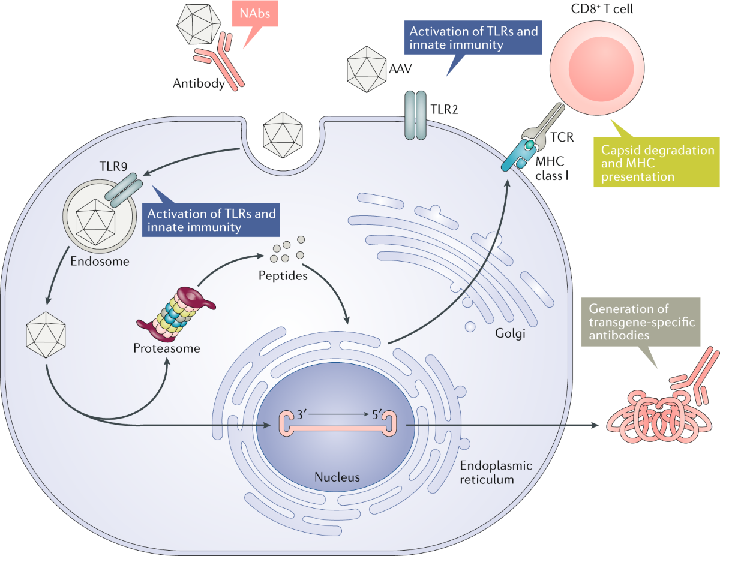
Adeno-Associated Viral (AAVs) are used to deliver smaller DNA packages and are a popular choice of gene therapy because it is safer and more efficient. AAVs do not combine into the host genome, their efficacy is in dividing cells. AAVs also prevail in the body for a long time. Another characteristic is that their efficacy can be impaired by antibodies and limited transport capacity. It can be used to treat several inherited diseases, neurological disorders, and ophthalmic diseases.
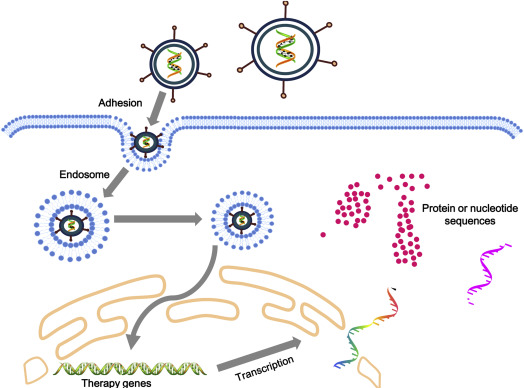
Adenoviral Vectors were introduced in the 1990s and are similar to AAVs, but they can be used for non-dividing cells and transport larger DNA packages . It would possibly cause stronger immune responses. Their disadvantages are the strong immune responses and their little persistence in the body. Adenoviral Vectors can be used in cancer immunotherapy, vaccines, and gene therapy for non-dividing cells.
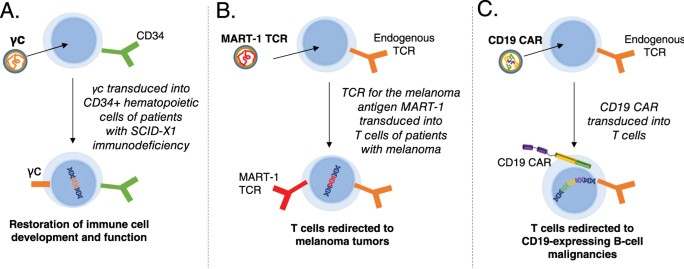
Lentiviral and Retroviral Vectors can transport larger RNA packages and combine into the host genome, fitting them for dividing cells. However, some disadvantages are the safety, as it is derived from HIV-1 and it is limited only to diving cells. It can be used for treating hematological disorders and cancer immunotherapy.
Overall viral vectors have limitations, such as the precision due to the locations, causing unplanned consequences, another limitation is the expensive and complex production of large-scale safe viral vectors, also the antibodies can impair the AAVs' efficacy leading to immune responses.
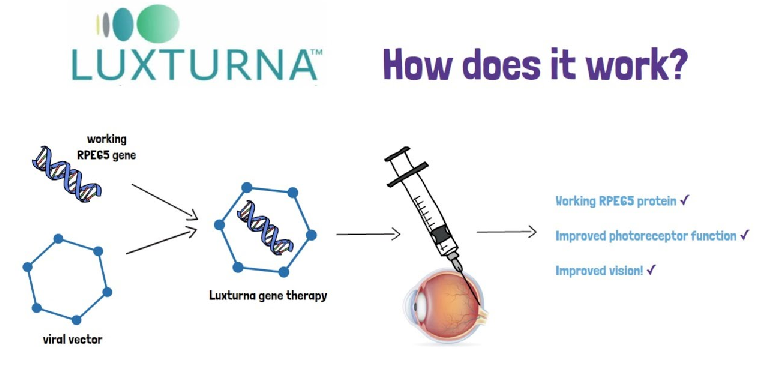

Gene therapy has various approved products and a variety of delivery vectors. Some Approved Products such as Zolgensma is used to treat spinal muscular atrophy; Luxturna which treats Leber congenital amaurosis; Spinraza, which treats spinal muscular atrophy; Kymriah and Yescarta, are used to treat certain types of leukemia and lymphoma. Some approved viral vectors are Adeno-associated viruses, Lentiviruses, and Adenoviruses, and the non-viral vectors are Liposomes and Nanoparticles.
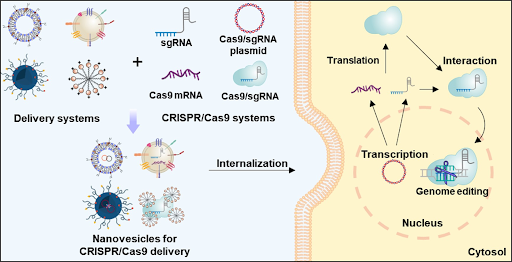
Gene therapy has some challenges. One of them is the delivery of the gene because it is difficult to ensure the gene was safely and correctly delivered to the cell. Another challenge is the immune response. The body may recognize the delivery vehicle or the gene as a strange body and attack it. It is necessary to highlight the possible side effects and the possibility of damage to the body, because some technologies, like CRISPR/Cas9, are not assured to insert the gene correctly. The protease Cas9 may cut a not desired part of the DNA.
References:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
https://my.clevelandclinic.org/health/treatments/17984-gene-therapy
https://medlineplus.gov/genetics/understanding/therapy/procedures/
https://patienteducation.asgct.org/gene-therapy-101/vectors-101
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7868676/
